|
Over the past three years the PET camera installed at the tumour
therapy facility in GSI has proven its capability of contributing
to the quality assurance of 12C radiotherapy [1].
In this report, we present the first studies concerning the PET camera
design and implementation onto a
dedicated hospital-based ion beam facility for cancer therapy,
proposed to be built in Heidelberg [2] due to the promising results
achieved with the GSI pilot project [3].
In the proposed facility,
due to the presence of a rotating gantry which will deliver the heavy
ion beam - thus satisfying an important medical need [4],
the flexibility of the PET camera must be enhanced so that it does
not collide with the patient or the couch nor with the beam gantry, as well as
it allows a fast access of the physicians to the patient.
Figures 1 and 2 depict possible PET scanner implementations. In Fig. 1,
the scanner moves
along the patient couch and, thus, can be positioned around the region
being irradiated. An aperture on the tomograph ring allows the beam to
pass through without touching the g-ray detectors. This configuration
can provide a full coverage of the volume under observation if the scanner
rotates 180° around its axial direction (ZPET)
during the beam extraction cycle (spill off, ~ 2 s). This detail, besides
being relevant for the image quality, is also important for a quantitative
analysis of the measured b+-activity.
If typical dimensions of a PET scanner are applied, an aperture of 30 cm for
the beam is considered (param. 7) and the distance between the scanner and
its support ring is 40 cm (param. 10), the map plotted in Fig. 3 is obtained.
The black and dark grey areas correspond to beam gantry and patient couch
angle combinations not suitable for therapy because the beam penetrates
the patient through the trunk of the body (caudo-cranial direction). The
angle combinations mapped in light grey are free for irradiation only
if the beam leaving the target volume does not activate substantially
the scanner support structure. In this configuration the scanner never
approaches the patient.
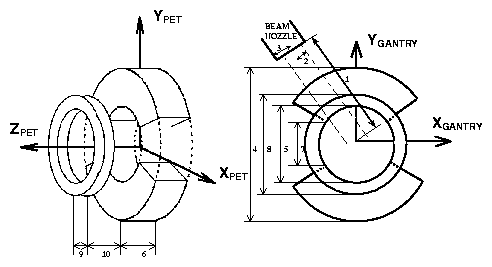
Fig. 1
PET scanner connected with the patient couch. The couch lies
along ZPET and the camera rotates, before the beginning of
the treatment, facing the beam with its aperture (not shown).
Legend: 1) Nozzle distance to isocenter, 2) Beam radius,
3) Nozzle radius,
4) Scanner outer radius, 5) Scanner inner radius, 6) Scanner width,
7) Scanner aperture for beam, 8) Support ring outer radius,
9) Support ring width and 10) Distance between scanner and its support
ring.
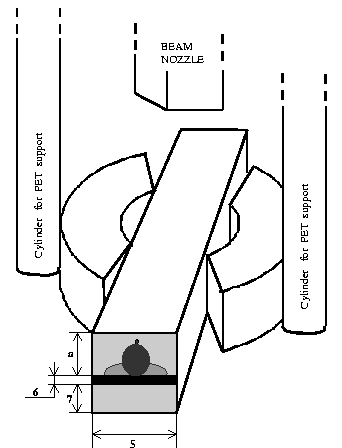 Fig. 2
PET scanner connected with the gantry, placed perpendicular to the beam
nozzle. The central parallelepiped represents the volume reserved for
patient and couch. Legend: 1) through 4) as in Fig. 1.
5) Couch width. 6) Couch
thickness. 7) Vertical range. a) couch width/2
Fig. 2
PET scanner connected with the gantry, placed perpendicular to the beam
nozzle. The central parallelepiped represents the volume reserved for
patient and couch. Legend: 1) through 4) as in Fig. 1.
5) Couch width. 6) Couch
thickness. 7) Vertical range. a) couch width/2
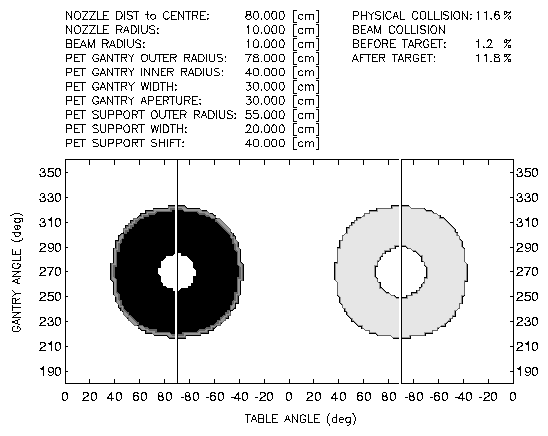
Fig. 3 Collision study for the case PET connected
with the patient couch. Black area: physical collision between beam gantry
and scanner. Dark grey area: beam collides with scanner support ring
before the target volume. Light grey area: as dark grey but
after irradiating the target volume.
In Fig. 2 the PET scanner is depicted, which is assumed to be
connected to the gantry and to be placed
parallel to the beam nozzle (the beam does not
collide with the scanner or its support structure
in any situation). A collision study yields a minimal
scanner aperture of 75 cm in order for
no physical collisions to occur between scanner and patient/couch.
If the scanner is placed parallel to
the beam nozzle the minimum aperture needed decreases to 65 cm
(the present dual-head PET scanner at the GSI therapy facility has
63 cm aperture).
These collision studies did not take into account
the movement of the scanner into the measurement position.
In addition to the collision studies summarized, we have developed
the tools to quantify the spatial resolution degradation as
one moves from a closed
ring to an open ring PET camera configuration: (i) a simulation
capable
of treating several
camera geometries and (ii) a flexible image reconstruction
routine being capable
of reading the output from the simulations. The reconstruction uses an
iterative procedure based on the maximum likelihood estimation
maximization algorithm. Due to the enormous amount of crystal combination
possibilities (over 150 million), dynamic memory allocation is used in
conjunction with a developed factorization scheme, which obliged
the routine to differ substantially from the one presently used at the
GSI therapy unit [5].
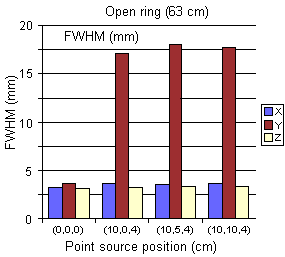
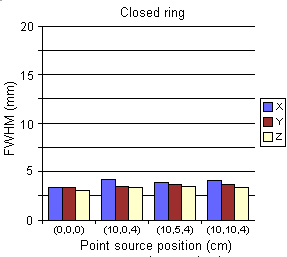
In Fig. 4 we depict the first results on the spatial resolution
degradation if one moves from a closed ring to an open ring detector
assembly. No Compton
scattering effects on the detector were simulated yet, the attenuation of
the g-rays in the crystals and thus the depth-of-interaction
influence on the spatial resolution was taken into account.
The degradation in
spatial information experimentally observed for detector crystals
coupled to photomultipliers according to the modified Anger
principle was also not included.
Fig. 4
Comparison between the spatial resolution achieved
with a closed ring versus an open ring positron camera
(diameter: 82.3 cm, aperture: 63 cm,
Fig. 1), built up of crystals of 5.0 × 4.5 mm2 frontal surface
and 30 mm depth.
1 Gesellschaft für Schwerionenforschung Darmstadt
References
|
[1]
|
W. Enghardt et al.,
GSI Scientific Report (1999) 164-5
|
|
[2]
|
K.D. Gross, M. Pavlovic (eds.), Proposal for a dedicated
ion beam facility
|
|
|
for cancer therapy, GSI Darmstadt, 1998
|
|
[3]
|
J. Debus et al., Strahlenther. Onkol., 176 Nr 5, (2000) 211-6
|
|
[4]
|
O. Jäkel and J. Debus,
Phys. Med. Biol. 45 (2000) 1229-41
|
|
[5]
|
K. Lauckner, Ph.D. Thesis, Dresden University of Technology,
1999
|
|