| Potential Extension of PET Technique to Proton Therapy Monitoring B | ||||||
|---|---|---|---|---|---|---|
| K. Parodi, W. Enghardt | ||||||
|
In perspective of the proposed proton and heavy ion (up to oxygen nuclei) therapy facility at Heidelberg [1] and on the basis of the positive impact of positron emission tomography to quality assurance of carbon ion therapy at GSI Darmstadt [2], we investigated the potential of PET for proton therapy monitoring [3].
We simulated by means of the FLUKA Monte Carlo code the b+-activation of a 9×9×30 cm3 PMMA target (C5H8O2, r = 1.19 g/cm3) irradiated by monoenergetic pencil-like proton beams (FWHM = 10 mm) in the energy range of interest for therapy (70 - 200 MeV).
The selected target contains the most abundant elements of the human body and is very suitable for experimental investigations.
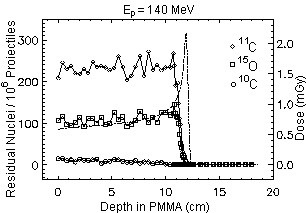
The simulated yield of b+-emitters of main relevance for the PET monitoring, namely 11C, 15O and 10C,
and the outcoming activity in 5 minutes (as a typical irradiation time) were
found one order of magnitude lower than those induced by carbon ion irradiation at the same range and number of projectiles. But
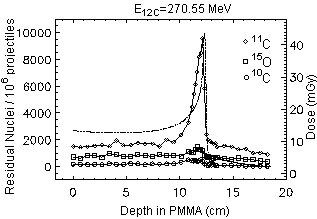
keeping into account the @ 20 times higher fluence of protons required in order to deliver the same physical dose and, furthermore, the lower relative biological effectiveness of protons in comparison to carbon ions, the activation induced by protons is expected to be at least twice as intense than for carbon ions. However, the spatial correlation between the positron emitter distribution and dose is poorer for protons, since they cannot experience the projectile fragmentation reaction leading to the sharp activity peak close to the dose maximum in the carbon ion case (Fig. 1, right).
Nevertheless, the range and Bragg peak position of protons are still correlated to the distal edge of the b+-emitter depth distribution (Fig. 1, left), depending on the energy threshold of the fragmentation reactions and on the O/C ratio of the irradiated target. Therefore, an important check of particle range and dose localisation seems to be possible for proton irradiation, too.
Fig. 1 Simulated spatial depth distribution of b+-emitters produced by 106 proton and carbon ion projectiles. The dashed-dotted line displays the dose profile. The factor of about 20 between the amount of dose delivered by the same number of primary particles can be seen. References
[1] K. D. Gross and M. Pavlovic (eds), Proposal for a
dedicated ion beam
|