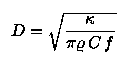|
The goal of this application of infrared radiation is the microspectroscopy of
organic components and their heavy metal complexes in solid or liquid environmental samples.
The absorption bands due to the
vibrational transitions are in the wavelength region of
l = 2-200 mm (5000-50 cm-1). The absolute amounts of the
components are very small. The concentration of solved organic
components in environmental water samples is usually lower than 10-6 Mol/l.
The conventional infrared spectrometry requires a particular sample preparation.
Different measuring techniques are known, but the sensitivity and the position
resolution are not satisfactory.
In order to improve the sensitivity of infrared measurements we are developing a thermo
optical detection method based on the mirage effect [1]. A solid sample is
periodically irradiated by a beam of monochromatic light produced by the
free-electron laser (FEL) CLIO at Lure/Orsay in France. An absorption induces
a temperature gradient which leads to an increase of the refractive index gradient.
This deflects a probe beam (i. e. He-Ne laser) propagating along the surface of the
sample (mirage effect). The periodic deflection of the probe beam is monitored by the use
of a high resolution position detector placed at a suitable distance away from the
illuminated sample. The magnitude of this periodic deflection can then be related to the
absorption coefficient of the material under investigation. This results in an
extremely high sensitivity (absorbances as low as 10-6 to 10-8 have been
measured in the visible light by this method).
We have carried out absorption measurements in the wavelength range from 9 mm up
to 40 mm with different samples. In these measurements we could reproduce some
features of the absorption of the samples, corresponding to the FTIR spectra.
With the incident energy being modulated, the heat generation will show a
corresponding time dependence.
A temperature profile then develops in the sample via heat diffusion.
For a heat source with a modulation frequency f the heat diffusion can be
described by the thermal diffusion length
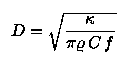
k denotes the thermal conductivity, r the material density and
C the specific heat of the sample.
Only heat generated within one thermal diffusion length will be able to reach
the surface with an appreciable magnitude.
For SiO2 one finds D ~ 1.5 mm for f = 1 MHz.
This result means that if the signal is measured about 1 ms after the
beginning of the laser pulse, the heat had time to diffuse only over a length
shorter than 2 mm. Therefore, one can
hope for a spatial resolution much smaller than the FEL wavelength, thus allowing
eventually microspectrometry. In that case the He-Ne laser would have to be
focused on a sufficiently small spot, which
has not yet been done in the present, preliminary, experiments.
We started first resolution test experiments with synthetic samples in May and October
2000. For this runs we prepared samples with the IR absorption in a controlled manner. The
pellets are made from KBr as matrix with homogeneous inclusions of interested materials
(humic acid). Furthermore, we measured one natural sample of calcite
containing humic acid inclusions.
We have carried out absorption measurements in the wavelength range from 9 mm up
to 15 mm (1100-670 cm-1).
The average IR-power of CLIO was in the order of 1 W over the wavelength range.
The laser time structure contains 10 ms trains (consist of short micropulses,
which have a duration of < 10 ps and are separated by intervals of 16 ns) with 25 Hz
repetition rate. For the measurement of the absorbance we have averaged the maximum
deflection of the He-Ne laser beam over 20 macropulses at each wavelength.
In different measurements we could reproduce the
structure of the absorption spectra of the samples, corresponding to the FTIR spectra.
|
|
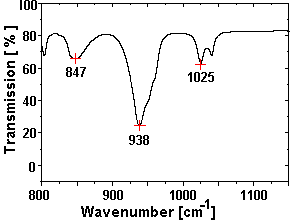
Fig. 1 FTIR spectrum of the test sample
(the substructure in the peak at 938 cm-1 is smeared out due to the
reduced resolution of this measurement).
|
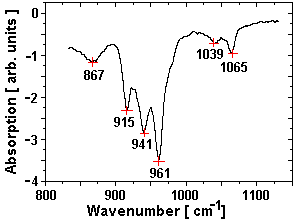
Fig. 2 Spectrum obtained by the mirage effect at the CLIO FEL.
|
Fig. 1 shows the FTIR-spectrum of a test sample (0.74 mg
UO2 (NO3)2 ×6 H2O in 300 mg KBr-pellet)
measured with a Perkin-Elmer Mod 2000 spectrometer, the spectrum measured at the CLIO
FEL is shown in fig. 2.
The splitting of the wide absorption peak around 941 cm-1 into tree peaks was not
observed in the samples with lower concentrations of nitrate and will be investigated
more detailed in near future.
In a next series of FEL experiments we would like to test this method at
longer wavelength in order to show that it will be a good
possibility for spectroscopic investigation with spatial
resolution. Preliminary results have already been obtained on Teflon between
25 mm and 40 mm
showing the applicability of the method at long wavelengths.
1 Institute of Radiochemistry
2 LURE, Orsay, France
References
[1] A.C. Boccara, D. Fournier, and J. Bodez, Appl. Phys. Lett. 36, 130 (1980)
IKH
05/28/01
© W. Seidel
|