RES³T - Rossendorf Expert System for Surface and Sorption Thermodynamics
|
Conversion to zero ionic strengthProtolysis constants (pK1/2) values of surface complexation reactions are usually determined at a constant ionic strength of a certain background electrolyte. Note, that the protolysis constants in RES³T are handled for the corresponding deprotonation reactions:
≡XOH2+ ↔ ≡XOH + H+ pK1
That might be different to the way as it is given in the original reference. To use the collected SCM data in speciation codes that support Surface Complexation Modelling (e.g. PHREEQC or Geochemist's Workbench), a conversion procedure has been integrated into RES³T, to calculate the pK1/2 values at zero ionic strength using the Davies equation (1,2) (Davies 1962). This equation is valid up to an ionic strength of I = 0.5 mol L−1. 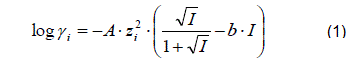
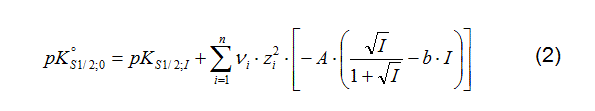
The parameters A and b (A = f(T,p), b = free parameter) are set to:
The pK1/2 conversion to zero ionic strength and the pK1/2 normalisation to a SSD = 2.31 nm−1 are seperate and not cummulative. |


