Chemical Speciation of dissolved matter
What means "Chemical Speciation" ?
Speciation is defined to be the distribution of one or more chemical elements amongst all its possible compoundsa (species) in a given systemb. The experimental determination of this distribution is sometimes also called speciation. An example for a simple system, the aqueous solution of uranyl phosphate in contact with air (with CO2) and all minerals that may precipitate, is shown in the next figure.
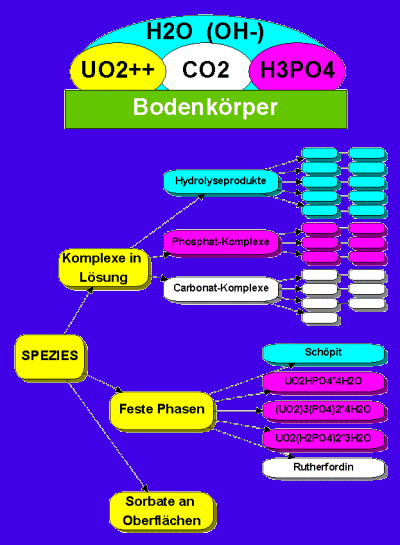
Possible species in the system
UO2-PO4-CO3-OH-H2O
Why are speciations so important?
The speciation determines whether a contaminant is easily transported and taken up by living matter, or can be retarded by precipitation or sorption processes.
What purposes are speciations used for?
- to prepare time-consuming or expensive experiments, e.g., X-ray or laser spectroscopy, in order to ensure conditions that avoid precipitations and maximize the proportion of the most interesting species.
- to analyze samples of mine and tailing waters: In which form do contaminants (U, As, Cd, Pb) occur, how are they transported?
- to model the risk assessment for the flooding of uranium mines (Königstein): What are the effects of rising pH and decreasing oxygen content on the speciation of radium and uranium?
- to estimate the effect of phosphate addition to tailing pond waters of uranium milling facilities (Freital) on the uranium migration behavior
Which factors determine the species distribution in natural systems?
- temperature and pressure
- concentrations of the elements
- ionic strength (activity coefficients !)
- pH value
- redox state
- present solid phases
- type, size and structure of available surfaces
- colloids
- micro organisms
Fundamentals
All the above mentioned factors are connected through thermodynamic laws. They mutually effect each other, the overall system is a very complex one.
Not always it is justified to assume thermodynamic equilibrium in natural systems. Especially problematic cases involve reactions with solid phases or redox processes. What does this mean?
- Often the primarily precipitating minerals are only metastable and slowly transform into more ordered, crystalline phases.
- Many minerals are weathering when in contact to water or air, secondary solid phases may form as a result.
Such comparatively slow reactions require to consider kinetic rate laws. The influence of possibly precipitating phases on the uranium speciation is illustrated in the following two figures, the chemical system is the example from the beginning of this document.
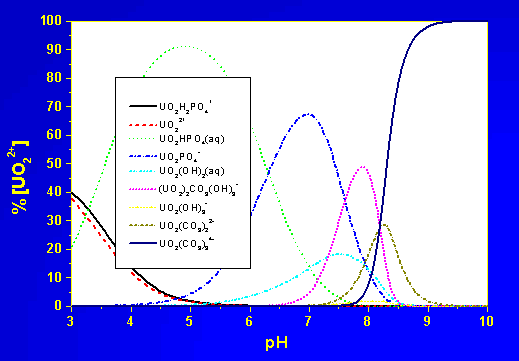
Uranium speciation in the example system
UO2-PO4-CO3-OH-H2O at
oversaturation
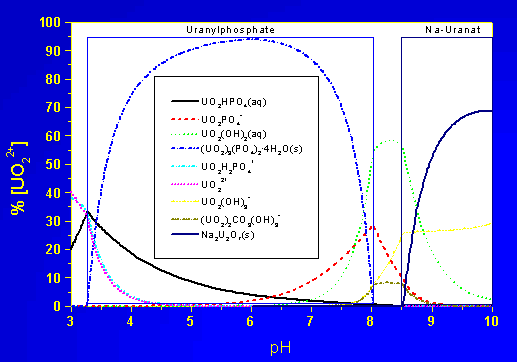
Uranium speciation in the example system
UO2-PO4-CO3-OH-H2O
with mineral precipitation
Thermodynamic laws are all based on activities rather then concentrations, activity coefficients are necessary to mutually transform these two quantities. These activity coefficients can be calculated from different models that depend on the ionic strength of the solutions. Examples for such models are: (extended) Debye-Hückel, Davies, S.I.T., Pitzer.
Speciation modeling
The mathematical treatment of speciation problems yields nonlinear equation systems that can only be solved iteratively. Usually, the numerics consists of two steps. First, initial estimates for all concentrations are computed and fed into a robust minimization routine. The results form this step are then refined with a fast hybrid Newton-Raphson method with relaxation. During each iteration step the activity coefficients are kept constant, they are updated after the computation of all concentrations at the end of each iteration step.
The overall problem is a challenge both from a chemical and from a numerical point of view. Thus, the application of computers is a natural consequence!
Typical results of speciation modeling efforts are the species distributions for all elements, their activity and activity coefficients, the system pH, redox potentials, and saturation indices for all possible minerals.
Software for chemical speciation modeling
The following list of software packages used for speciation modeling is far from being thorough
- EQ3/6 (WOLERY, 1992)
- the PHREEQE family (PARKHURST et al., 1980) with HARPHRQ (BROWN et al., 1991), PHRQPITZ (PLUMMER et al., 1988) and PHREEQC (PARKHURST, 1995)
- MINEQL (WESTALL et al., 1976) and its successors HYDRAQL (PAPELIS et al., 1988), MINTEQA2 (ALLISON et al., 1991) and MINEQL+ (SCHECHER, 1995)
- WATEQ (TRUESDELL & JONES, 1973) with WATEQ4F (BALL et al., 1981) and WATEQP (APPELO & POSTMA, 1994)
- SOLMINEQ.88 (KHARAKA et al., 1988)
- CHEMSAGE (ERIKSSON & HACK, 1990)
- C-HALTAFALL (ÖSTHOLS, 1994)
Criteria for the selection of appropriate speciation modeling software
In order to choose the modeling software most appropriate for a specific speciation problem various evaluation criteria may be applied:
- Is there a manual and the source code available, is external support provided?
- What is the maximum problem dimension (number of species, heterogeneous phases and reactions) the software can deal with?
- Does the software take into account redox reactions, sorption processes, and kinetics?
- Are solid phases / minerals considered?
- Which models for activity coefficients are implemented?
- Which numerical methods are used, how fast and stable are they?
- Other details to be cared of: upper concentration limits, check of charge imbalances, required quality of initial estimates, convergence criteria for the iteration, effects of varying temperature or pressure.
- Which databases can be used and how? Is it possible to introduce own changes or additions to these databases?
- Is there a graphical output integrated?
- Which operation system and programming language is used?
Transport modeling
In many real-world applications the speciation itself is only a module inside an even more complex software suite. There, also the spatial and temporal components of contaminant migration are computed, i.e., species distributions as functions of time and space coordinates. Besides profound chemical knowledge, such models require expertise from filed such as geology or hydrodynamics. This leads to coupled or reactive transport models.
Here, two approaches are wide-spread. Either, the equation system for the speciation calculations is directly substituted into the transport equation, or alternating speciation and transport is computed for each iteration step, keeping constant the respective other parameters.
Examples for such coupled transport codes are given below, again the list is not exhaustive:
- CHEMTARD (BENETT et al., 1992)
- OS3D/GIMRT (STEEFEL & YABUSAKI, 1995)
- CHEQMATE (HAWORTH et al., 1988)
- CHMTRNS (NOORISHAD et al., 1987)
- THCC (CARNAHAN, 1987)
- HYDROGEOCHEM (YEH & TRIPATHI, 1990)
- TReAC (NITZSCHE, 1997)
- COTAM (HAMER & SIEGER, 1994)
- UNSATCHEM-2D (SIMUNEK & SUAREZ, 1993)
- MT3DMS (ZHENG & WANG, 1998)
Databases
Databases are inherent components of every speciation modeling. Thermodynamic databases supply the equilibrium constants for all chemical reactions to be considered in a given system, so they define the extend to which each possible species is actually formed under the encountered conditions. Kinetic data are the precondition for the description of the time dependence of any chemical reaction. Databases can be implicitly incorporated into the source code of a program (the most inflexible method), they can be linked to the program over external modules, or they can be delivered by the user by means of input file specifications. Often, external databases must be converted from their standard format to the special conventions required by the speciation software, a time-consuming and error-prone process. Wide-spread thermodynamic databases are listed below:
- EQ3/6 set (Lawrence Livermore National Laboratory - LLNL) with NEA, SUP, CHV, COM, HMW, PIT, ALT: of varying coverage and quality, but centrally maintained, well documented and thus judgeable
- HYDRAQL / MINEQL+ / MINTEQA2: very comprehensive (especially radionuclides), but insufficiently documented and not centrally maintained.
- Others: HATCHES, WATEQ, CHEMVAL, SGTE
When dealing with actual real-world cases, most often the available database is insufficient in scope and quality. This calls for the integration of own experimental findings (or form other scientists) in order to close the database gaps at least partially. Such experiments may yield:
- solubility products
- complex stability constants (from potentiometry or spectroscopy)
- parameters of surface complexation or ion exchange
A general problem associated with sorption and kinetics is the lack of comprehensive databases. One reason may be the high efforts (in time) to generate such data sets.
Assessment criteria for databases
Again, the selection of appropriate thermodynamic databases cab be guided by various evaluation criteria:
- What is the scope and focus of the database, which species are included?
- What is the internal data format?
- Was there a critical data assessment procedure?
- Are the data entries internal consistent?
- Are there double entries or obvious errors?
- Are the data sources / original references documented?
- Is it possible to change, add, remove data entries?
- Which models for activity coefficients and temperature dependence or supported ?
- Are data for sorption or kinetics included?
Examples for speciation calculations:
All computations are based on the EQ3/6 package from the LLNL.
![Mineralization in the Königstein mine as f([UO2+2], [PO4-3], pH)](/FWR/SPEZI/KS_3D.gif)
a) Mineral precipitation in the 4th aquifer of the former uranium mine Königstein / Saxony as
function of pH, uranium content and phosphate content
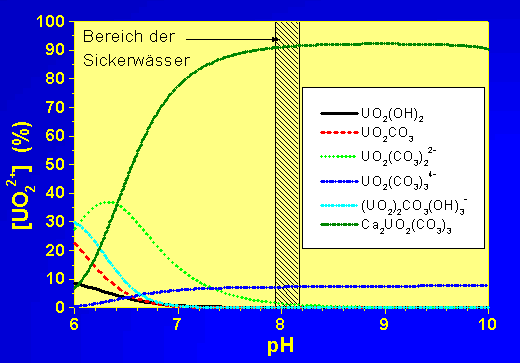
b) Uranium speciation in seepage waters from uranium mining rock piles
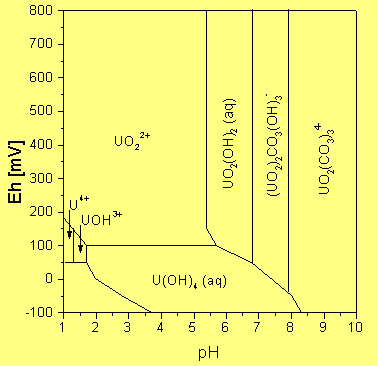
c) Eh-pH diagram of uranium at 0.3 % CO2 in the air
Summary
The computation of species distributions is essential for many tasks. It is a complex challenge when dealing with natural systems!
Before starting the computer the user has to perform a lot of brain work: How can the problem be formulated in terms of chemical components and physico-chemical processes?
There is neither an ideal speciation software nor a perfect database. Depending on the actual problem to be solved the most appropriate software and database must be selected and checked!
Modeling, sampling, chemical analysis, laboratory experiments and database maintenance are all part of an iterative scheme, they are mutually dependent and must be critically verified!

